J. Roales, J.M. Pedrosa, P. Castillero, M. Cano, T.H. Richardson, A. Barranco, A.R. González-Elipe
ACS Applied Materials and Interfaces, 4 (2012) 5147-5154
doi: 10.1021/am3010169
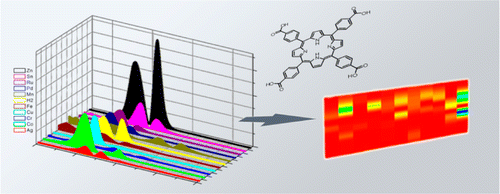
In this work, the carboxylic acid derivatives of a free-base porphyrin, 5,10,15,20-tetrakis(4-carboxyphenyl)-21H,23H-porphyrin, and 10 of its metal derivatives (TCPPs) have been used for optical gas sensing. For this purpose, microstructured columnar TiO2 thin films prepared by GAPVD (glancing angle physical vapor deposition) have been used as host materials for the porphyrins as they are non–dispersive and porous, allowing their use for UV–visible spectroscopy and gas sensing. The chemical binding between the dye molecules and the TiO2 has been studied through infrared spectroscopy, and the obtained spectral changes have been found to be compatible with chelating and/or bidentate binding modes of the carboxylate groups on the TiO2 surface. When hosted in the film, the UV–visible spectra of the porphyrins featured a blue shift and broadening of the Soret band with respect to the solution, which has been attributed to the formation of π–π aggregates between porphyrin molecules. The composite porphyrin/TiO2 films obtained from each of the 11 porphyrins have been exposed to 12 different volatile organic compounds (VOCs), and their respective gas–sensitive properties have been analyzed as a function of the spectral changes in their Soret band region in the presence of the analytes. The set of composite films has shown high selectivity to the analyzed volatile compounds. For each analyte, an innovative way of showing the different responses has been developed. By means of this procedure, an imagelike recognition pattern has been obtained, which allows an easy identification of every compound. The kinetics of the exposure to several analytes showed a fast, reversible and reproducible response, with response times of a few seconds, which has been attributed to both the sensitivity of the porphyrins and the high porosity of the TiO2 films. Also, increasing concentrations of the analytes resulted in an increase in the magnitude of the response, indicating that the sensor behavior is also concentration-dependent.

