C. Sánchez-Sánchez, N. Orozco, J.P. Holgado, S.K. Beaumont, G. Kyriakou, D.J. Watson, A.R. González-Elipe, L. Feria, J. Fernández-Sanz, R.M. Lambert
Journal of American Chemical Society, 137 (2015) 940–947
doi: 10.1021/ja5115584
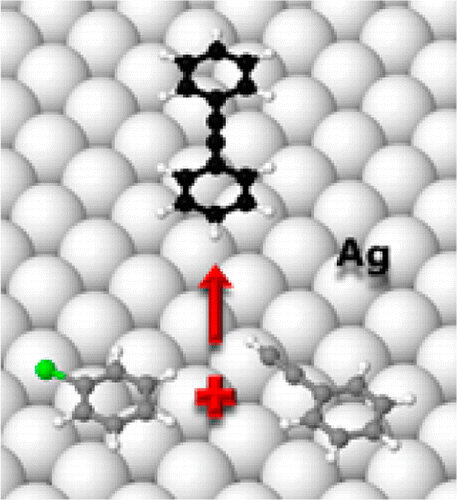
Scanning tunneling microscopy, temperature-programmed reaction, near-edge X-ray absorption fine structure spectroscopy, and density functional theory calculations were used to study the adsorption and reactions of phenylacetylene and chlorobenzene on Ag(100). In the absence of solvent molecules and additives, these molecules underwent homocoupling and Sonogashira cross-coupling in an unambiguously heterogeneous mode. Of particular interest is the use of silver, previously unexplored, and chlorobenzene—normally regarded as relatively inert in such reactions. Both molecules adopt an essentially flat-lying conformation for which the observed and calculated adsorption energies are in reasonable agreement. Their magnitudes indicate that in both cases adsorption is predominantly due to dispersion forces for which interaction nevertheless leads to chemical activation and reaction. Both adsorbates exhibited pronounced island formation, thought to limit chemical activity under the conditions used and posited to occur at island boundaries, as was indeed observed in the case of phenylacetylene. The implications of these findings for the development of practical catalytic systems are considered.