K. Brandt, M.E. Chiu, D.J. Watson, M.S. Tikhov, R.M. Lambert
Journal of Physical Chemistry C, 116 (2912) 4605-4611
doi: 10.1021/jp208831h
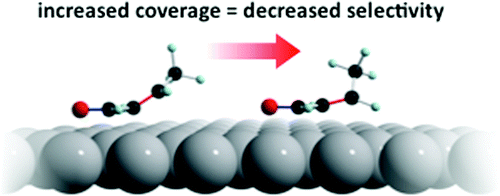
The chemoselective hydrogenation of crotonaldehyde to crotyl alcohol was studied by temperature-programmed desorption/reaction, high-resolution XPS, and NEXAFS. The organic molecule adsorbed without decomposition, all three possible hydrogenation products were formed and desorbed, and the clean overall reaction led to no carbon deposition. Selectivities up to 95% were found under TPR conditions. The observed behavior corresponded well with selectivity trends previously reported for Ag/SiO2 catalysts, and the present findings permit a rationalization of the catalytic performance in terms of pronounced coverage-dependent changes in adsorption geometries of the reactant and the products. Thus, at low coverages, the C═O bond in crotonaldehyde lies almost parallel to the metal surface, whereas the C═C was appreciably tilted, favoring hydrogenation of the former and disfavoring hydrogenation of the latter. With increasing coverage of reactants, the C═C bond was forced almost parallel to the surface, rendering it vulnerable to hydrogenation, thus markedly decreasing selectivity toward formation of crotyl alcohol. Butanol formation was the result of an overall two-step process: crotonaldehyde → crotyl alcohol → butanol, further hydrogenation of the desired product crotyl alcohol being promoted at high hydrogen coverage due to the C═C bond in the unsaturated alcohol being driven from a tilted to a flat-lying geometry. Finally, an explanation is offered for the strikingly different behavior of Ag(111) and Cu(111) in the chemoselective hydrogenation of crotonaldehyde in terms of the different degrees of charge transfer from metal to C═O π bond, as suggested by C 1s XPS binding energies.

