P. Castillero, J.R. Sánchez-Valencia, M. Cano, J.M. Pedrosa, J. Roales, A. Barranco, A.R. González-Elipe
ACS Applied Materials and Interfaces, 2 (2010) 712-721
doi: 10.1021/am900746q
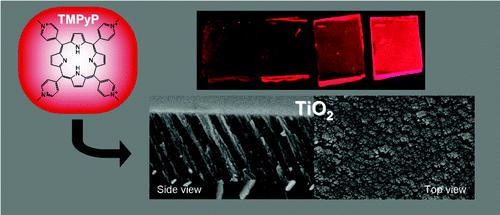
Fluorescent tetracationic porphyrin (TMPyP) molecules have been incorporated into optically transparent TiO2 thin films acting as a host material. The films, with a columnar structure and open pores, were prepared by electron evaporation at glancing angles (GAPVD). The open porosity of the films has been estimated by measuring a water adsorption isotherm with a quartz crystal monitor. TMPyP molecules were infiltrated in the host thin films by their immersion into water solutions at controlled values of pH. The state of the adsorbed molecules, the infiltration efficiency, and the adsorption kinetics were assessed by analyzing the optical response of the films by UV−vis absorption and fluorescence techniques. The infiltration efficiency was directly correlated with the acidity of the medium, increasing at basic pHs as expected from simple considerations based on the concepts of the point of zero charge (PZC) developed for colloidal oxides. By a quantitative evaluation based on the analysis of the UV spectra, the infiltration process has been described by a Langmuir type adsorption isotherm and an Elovich-like kinetics. The accessibility of the infiltrated molecules in the TMPyP/TiO2 composite films is assessed by following the changes of their optical properties when exposed to the acid vapors and their subsequent recovery with time.

