Ioannis V. Yentekakis, Grammatiki Goula, Paraskevi Panagiotopoulou, Stavroula Kampouri, Martin J. Taylor, Georgios Kyriakoub, Richard M. Lambert
Applied Catalysis B: Environmental 192, 357 (2016)
doi: 10.1016/j.apcatb.2016.04.011
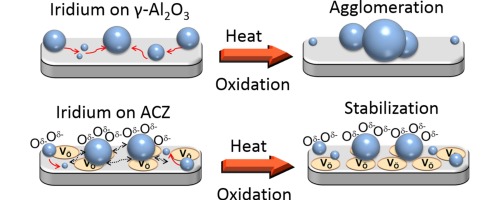
Iridium nanoparticles deposited on a variety of surfaces exhibited thermal sintering characteristics that were very strongly correlated with the lability of lattice oxygen in the supporting oxide materials. Specifically, the higher the lability of oxygen ions in the support, the greater the resistance of the nanoparticles to sintering in an oxidative environment. Thus with γ-Al2O3 as the support, rapid and extensive sintering occurred. In striking contrast, when supported on gadolinia-ceria and alumina-ceria-zirconia composite, the Ir nanoparticles underwent negligible sintering. In keeping with this trend, the behavior found with yttria-stabilized zirconia was an intermediate between the two extremes. This resistance, or lack of resistance, to sintering is considered in terms of oxygen spillover from support to nanoparticles and discussed with respect to the alternative mechanisms of Ostwald ripening versus nanoparticle diffusion. Activity towards the decomposition of N2O, a reaction that displays pronounced sensitivity to catalyst particle size (large particles more active than small particles), was used to confirm that catalytic behavior was consistent with the independently measured sintering characteristics. It was found that the nanoparticle active phase was Ir oxide, which is metallic, possibly present as a capping layer. Moreover, observed turnover frequencies indicated that catalyst-support interactions were important in the cases of the sinter-resistant systems, an effect that may itself be linked to the phenomena that gave rise to materials with a strong resistance to nanoparticle sintering.