A. Gómez-Ramírez, V.J. Rico, J. Cotrino, A.R. González-Elipe, R.M. Lambert
ACS Catalysis, 4 (2014) 402-408
doi: 10.1021/cs4008528
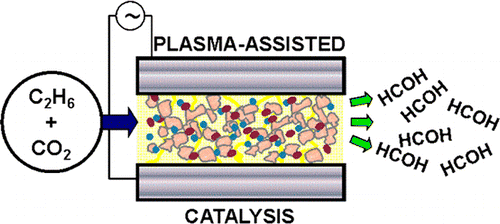
Plasma-assisted catalysis of the reaction between CO2 and C2H6 in a single-pass, ferroelectrically moderated dielectric barrier discharge reactor has been studied at near ambient temperature as a function of physicochemical and electrical reaction variables. The presence of small amounts of a vanadia/alumina catalyst dispersed on the BaTiO3 ferroelectric markedly enhanced the production of formaldehyde, the focus of this work. A maximum HCOH selectivity of 11.4% (defined with respect to the number of ethane carbon atoms consumed) at ∼100% ethane conversion was achieved, the other products being CO, H2O, H2, CH4 and a small amount of C3H8. N2O was also an effective partial oxidant (HCOH selectivity 8.9%) whereas use of O2 led to complete combustion, behavior that may be rationalized in terms of the electron impact excitation cross sections of the three oxidants. Control experiments with the coproducts CH4 and C3H8 showed that these species were not intermediates in HCOH formation from C2H6. Analysis of reactor performance as a function of discharge characteristics revealed that formaldehyde formation was strongly favored at low frequencies where the zero-current fraction of the duty cycle was greatest, the implication being that plasma processes also acted to destroy previously formed products. A tentative reaction mechanism is proposed that accounts for the broad features of formaldehyde production.

